Episode 201
The celebration of Hot Tea Month has been steadily gaining steam (pun intended). Marketers have invested in building momentum in recent years, coinciding with growing interest in wellness and healthy lifestyle habits. Many consumers, particularly in Western markets, are increasingly aware of these benefits and incorporating tea into their daily routines. | Cherry red erythrosine (known commercially as Red Dye No. 3) is widely used to color beverages, snack foods, and candy but is not commonly used to color tea. The US Food and Drug Administration (FDA) banned Red Dye No. 3 in cosmetics and personal care products out of concern for health risks. On Jan. 15, the FDA extended the ban to include food products, beverages, and ingested drugs. | Argentine tea produced in Misiones and northern Corrientes has been awarded a Geographical Indication (GI) from the European Union (EU). Tea from these provinces is the southernmost tea produced on the planet.

Tea News for the week ending 17 January 2025
Powered by RedCircle
India Tea News
Tea News

Marketers Add Momentum to Hot Tea Sales
By Dan Bolton
The celebration of Hot Tea Month has been steadily gaining steam (pun intended). Marketers have invested in building momentum in recent years, coinciding with growing interest in wellness and healthy lifestyle habits. Many consumers, particularly in Western markets, are increasingly aware of these benefits and incorporating tea into their daily routines.
Hot Tea Month has highlighted traditional black and green herbal teas in the past decade and newer and trendy varieties such as matcha, chai, and bubble tea. The month has become a time for people to explore new types of tea and experiment with different brewing methods and innovations to enhance tea’s cultural and health aspects.
Continued…
Technomic reports that chai, at 4.1%, was the fastest-growing tea category on menus in the US in 2023. In Canada, chai offerings grew by 25% over two years, with specialty tea menu items up by 19.8% and hot tea up by 18.3%.
Technomic asked consumers why they order hot tea away from home. The most common response was “to get a pick-me-up” at 23%, followed by “wash down my food” at 19% and relax or unwind with family and friends (18%). Survey respondents cited “quench my thirst” as the fourth most common reason to order tea, while 18% said they order tea with snacks or indulge in a dessert/treat.
Transparency Market Research (TMR) projects the tea market will expand to $33.9 billion by 2032, growing at a combined average rate of 5.9%. “Rising disposable incomes and growing health awareness in countries like Brazil, South Africa, and the Middle East present opportunities for expansion,” writes TMR.
Researchers also cite growing demand for premium teas such as single-origin, organic, and artisanal blends. Customers are willing to pay a premium for high-quality, sustainable, ethically sourced tea.
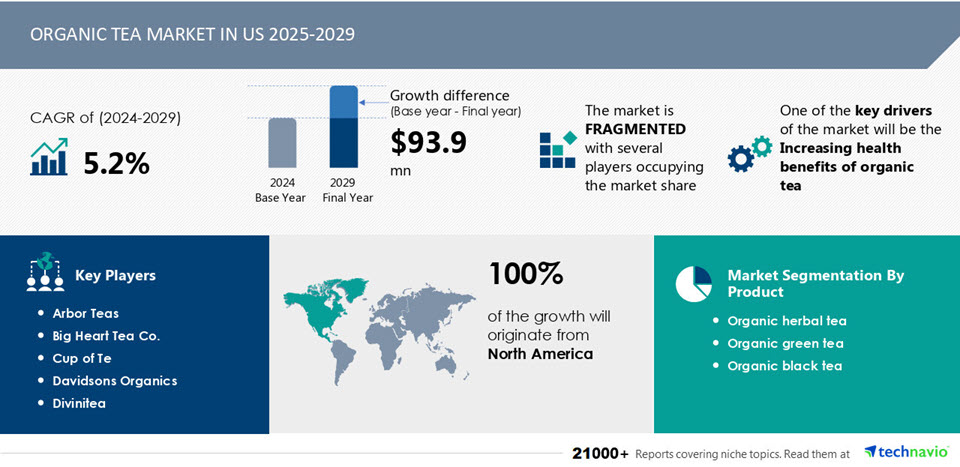
Technavio projects the US market for organic tea to grow by $93.9 million through 2029. Drivers include organic matcha and premium organic black tea, which are projected to grow at a 5.2% CAGR.
Technavio writes that consumers increasingly seek teas rich in antioxidants like epicatechin gallate and catechins. “organic matcha green tea is leading the trend.”

FDA Ban Imposed in 1990 Extended to Include Food and Beverages
By Dan Bolton
Cherry red erythrosine (known commercially as Red Dye No. 3) is widely used to color beverages, snack foods, and candy but is not commonly used to color tea.
In 1990, the US Food and Drug Administration (FDA) banned Red Dye No. 3 in cosmetics and personal care products. On January 15, 2025, the FDA extended the ban to include food products and ingested drugs.
Concerns were first raised about the safety of Red Dye No. 3 during the 1980s when studies linked it to thyroid tumors in laboratory animals. No conclusive studies have been conducted on the connection of dye to human cancer, but Australia, Japan, and the European Union have instituted bans out of caution.
Over the years, ongoing research identified additional potential health risks regarding its role in hormone disruption and cancer risk.
The 2025 ban reflects growing caution over the potential long-term effects of synthetic additives on health, particularly for children who are most likely to consume products containing the dye.
These products include maraschino cherries, PEZ candy, Brach’s candy corn, Jelly Belly candies, strawberry-flavored beverages, toaster pastries, ice pops, frozen fruit bars, the red icing on some cookies, and snack cakes.
Manufacturers must remove the dye from their products by January 2027. In recent years, formulators have replaced synthetic colors and additives with natural alternatives such as beet juice, red cabbage pigment, or carmine, a coloring made from insects.
BIZ INSIGHT — The National Confectioners Association expressed concerns about the cost of reformulations, given that Red Dye No. 3 has not been shown to cause human cancer. Jim Jones, the FDA’s deputy director for human foods, told NBC News that “the FDA cannot authorize a food additive or color additive if it has been found to cause cancer in humans or animals.” Once announced, the confectioners released this statement: “Food safety is the number one priority for US confectionery companies, and we will continue to follow and comply with FDA’s guidance and safety standards.”
Argentina’s Misiones Tea Region is Awarded GI Status
By Horacio Bustos
Argentine tea produced in Misiones and northern Corrientes has been awarded a Geographical Indication (GI) from the European Union (EU).
Tea from these provinces is the southernmost tea produced on the planet.
GI’s recognition of “Argentine Tea” protects the authenticity of its origin and attests to its quality worldwide, enhancing its cultural value and opening up opportunities in international markets.
Designations of origin and traditional production methods are part of a system that protects products tied to specific geographical areas. Introduced in 1992, the system began a broader effort to safeguard traditional foods and agricultural products from misuse and imitation.
It was the work of a whole year led by the General Directorate of Yerba Mate and Tea of the Ministry of Agriculture and Production, with the support of the Undersecretariat of Agri-Food Markets and International Insertion of the Secretariat of Agriculture, Livestock, and Fisheries of the Nation.
The effort was coordinated with the Argentine Tea Association, INTA Cerro Azul, and INTI Misiones. The presentation and defense of the request for the GI of Argentine tea before the National Advisory Commission on Geographical Indications and Denominations of Origin for Agricultural and Food Products was carried out virtually.
It is important to highlight that the GI is an intellectual property right that operates under the jurisdiction of the National Secretariat of Agriculture and is regulated by law.
Adriana Yánez, representing the Argentine Tea Association, detailed in her presentation the particularities of the tea from this region. These teas are notable for their high polyphenol content, which gives them antioxidant power; their liquor is reddish or coppery (in black tea) and bright yellow or greenish (in green tea), with good shine and transparency even in cold infusion.
The teas are balanced and smooth in flavor, with low to medium astringency and a certain sweetness, while floral and vegetal notes predominate in the aroma.
These qualities are achieved thanks to the orthodox production process, using rollers and rotor vanes, which has also contributed to its international recognition for its safety.
Studies characterizing Argentine products were also included to support the presentation, with comparisons of the distinct Misiones terroir with other teas worldwide and a compilation of publications and different essays.
BIZ INSIGHT – The first GI in the European Union was granted in 1992 to the French “Roquefort” cheese, a blue cheese made from sheep’s milk and produced in the Roquefort-sur-Soulzon region of southern France.
FEATURE
Lessons From Japan’s Deep Engagement with Tea
By Dan Bolton
In medieval times, Japanese commanders bestowed teaware on valiant survivors at banquets, explains historian Morgan Pitelka. Later, in the early modern period, tea culture permeated every walk of life in the imperial capital of Kyoto. The prevalence of Chanoyu in the Shogun era, a time of social upheaval and war, provides relevant insights into coping with stressful times today. Pitelka reveals tea’s unique role in Bringing Communities Together during war and peace.
Listen to the story
Powered by RedCircle
Sharing is Caring (please share this newsletter link with a friend in tea)
Hot Tea Gains Marketing Momentum | FDA Bans Red Dye No. 3 in Foods and Beverages | Argentina’s Misiones Tea Growing Region is Awarded Geographic Indication Status | Episode 201 | 17 January 2025
Sign up to receive Tea Biz weekly in your inbox.

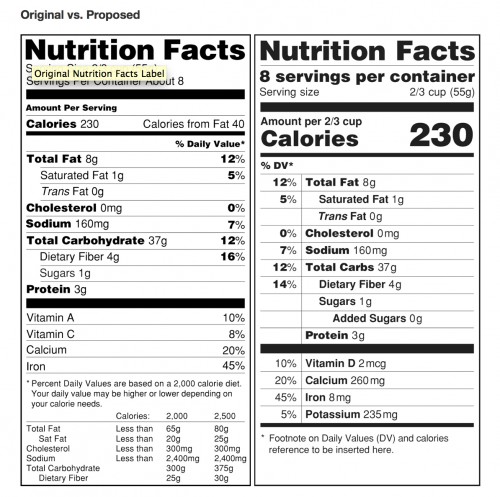 There are two fundamental parts to the proposed changes. The first addresses the actual nutritional information being reported. There would be a reevaluation of the daily nutritional values of certain vitamins and minerals. Vitamin D would be added, while Vitamins C and A would be eliminated. Additional information about sugar would be provided as well. Serving sizes would be most affected by the rules rewrite. The new serving sizes would better reflect how people actually eat and drink today. For example, a 20 ounce bottle of cola would no longer list the contents as two servings. A bottled drink that would usually be consumed during one sitting would need to have nutritional information reflect the values for the entire bottle. Larger bottles would list the amounts for a single serving, as well as the values if the entire bottle is consumed. The second change impacts the actual layout of the label. Calories would become more prominent. The chart showing daily nutritional values would be reversed so the percentages for each item would be listed before the actual amounts.
There are two fundamental parts to the proposed changes. The first addresses the actual nutritional information being reported. There would be a reevaluation of the daily nutritional values of certain vitamins and minerals. Vitamin D would be added, while Vitamins C and A would be eliminated. Additional information about sugar would be provided as well. Serving sizes would be most affected by the rules rewrite. The new serving sizes would better reflect how people actually eat and drink today. For example, a 20 ounce bottle of cola would no longer list the contents as two servings. A bottled drink that would usually be consumed during one sitting would need to have nutritional information reflect the values for the entire bottle. Larger bottles would list the amounts for a single serving, as well as the values if the entire bottle is consumed. The second change impacts the actual layout of the label. Calories would become more prominent. The chart showing daily nutritional values would be reversed so the percentages for each item would be listed before the actual amounts.